460
Views & Citations10
Likes & Shares
The role of Na+/K+-ATPase α subunit isoforms in brain tissue hydration and [3H]-ouabain binding to cell membrane were investigated in three age groups of rats. In brain cortex, subcortex and cerebellum tissues the variation of water content and [3H]-ouabain binding to cell membrane were defined by means of dose-dependent [3H]-ouabain solutions. The obtained results showed that the initial quantity of water in cortex tissues of all animal groups was higher than in subcortex and cerebellum tissues. Age-dependent tissue dehydration in all three zones of brain was due to inhibition of Na+/K+ pump activity. The study of [3H]-ouabain dose-dependent effect on brain tissues showed that the curves of hydration sensitivity mainly consisted of six components where the isoforms of high affinity (α3) were more sensitive. These results allow concluding that there may be minimum six metabolic mechanisms involved in cell volume regulation. The curves of dose-dependent ouabain binding consists of three components corresponding to Na+/K+ pump isoforms (α1, α2, α3). The age-dependency of these isoforms (mainly of high affinity ones - α3) were more expressed in brain cortex tissue of adult animals than in their subcortex and cerebellum tissues. The high affinity α3 isoforms were depressed in brain tissues of old rats. Thus, the obtained data allow concluding about the primary role of Na+/K+-ATPase high affinity α3 isoforms in brain tissue dehydration and ouabain binding.
Keywords: Rat, Brain, Na+/K+ pump, Na+/K+-ATPase, Hydration, [3H]-ouabain
INTRODUCTION
The important physiological role of water in cell functional activity is well known and widely accepted but its messenger role in generation of various diseases has not been paid an adequate attention to. It is also noted that ageing-induced nerve disorders are accompanied by tissue dehydration in living objects. The role of cell hydration in memory processes, as well as the role of cell volume controlling metabolic mechanisms in neuronal dysfunctions has not been investigated in detail.
Our early works have shown that cell hydration depends on the ratio of cell membrane proteins characterized by different properties [1-3]. Cell swelling (over hydration) increases the functionally active membrane receptors, while its shrinkage (dehydration) has opposite effect. On the basis of these data cell swelling has been considered as a primary messenger switching on the intracellular signaling system through which the protective reaction of cells is realized.
Among the number of mechanisms involved in cell volume regulation, Na+/K+ pump has the basic role [1-5]. The main pathways regulating its activity are the enzymatic systems connected with ATP-utilization and the ion transporting mechanism through Na+/Ca2+ exchange [5-7]. Detailed investigations have shown that this ionic exchange is regulated by means of Na+/K+-ATPase high affinity (α3) receptors, while the Na+/K+ pump activity - through low affinity (α1) isoforms [8-12].
Taking into account the fact that ageing [13,14] can be considered as a consequence of the Na+/K+ pump dysfunction leading to decontrolling of tissue hydration, the purpose of our study was to investigate the variation of brain tissue hydration and the role of Na+/K+-ATPase isoforms in three age groups of rats.
MATERIALS AND METHODS
Animals
The experiments were performed on three separate groups of albino rats: young (6 weeks old), adult (18 weeks old) and old (18 months old) weighing 35-40 g, 100-120 g and 250-300 g, respectively. The animals were regularly examined, kept under control of the veterinary in LSIPEC and reserved in a specific pathogen free animal room under optimum conditions of 12 h light/dark cycle at 22 ± 2°C and the relative humidity of 50%. They were fed ad libitum on a standard lab chow and water.
Chemicals
Tyrode’s physiological solution (PS) containing (in mM) 137 NaCl, 5.4 KCl, 1.8 CaCl2, 1.05 MgCl2, 5 C6H12O6, 11.9 NaHCO3, 0.42 NaH2PO4 and adjusted to pH 7.4 with NaOH was used. All chemicals were obtained from “Medisar” Industrial Chemical Importation Company (Yerevan, Armenia). The [3H]-ouabain with specific activity 25.34 Ci/mM (PerkinElmer, Massachusetts, USA) from 10-11 M to 10-4 M dissolved in PS was used for intraperitoneal (i/p) injections. The volume of injected solution was adjusted according to the weight of animals (0.02 ml/g).
Experimental design
The experiments were carried out in in vivo conditions on young, adult and old rats. In the first series of experiments the dynamics of hydration changes in brain tissues of all animal groups was investigated. For this the animals of young (n=3), adult (n=3) and old (n=3) groups were i/p injected by PS (as a control).
In the second series the data of the first series were compared with that of the second one. The new animal groups were chosen of the same number (n=3) and i/p injected by [3H]-ouabain at 10-4 M ouabain.
For the third series of experiments new three aged animal groups were taken and every group was divided into 8 subgroups (3 animals in each) according to each used ouabain dose. These animals were i/p injected by corresponding dose of [3H]-ouabain (from 10-11 M to 10-4 M). As a control 8 subgroups of young as well as old animals were chosen and i/p injected by PS.
Tissue preparation
It is well known that the anesthetics with different chemical and pharmacological profiles significantly affect metabolic processes, which play an important role in regulation of cell volume [15-17]. Therefore, after the 15th min of i/p injection the animals in each series of experiments animals were sharply immobilized by freezing method (dipping their noses into liquid nitrogen for 3-5 s) [18] and decapitated. After such a procedure the full absence of somatic reflexes on extra stimuli was recorded.
The tissues of brain cortex, subcortex and cerebellum were isolated. From each animal of all groups 4 samples of each tissue were taken (12 samples from each tissue), washed three times in PS at 20°C and weighed. The protocol of experiments was the same for young, adult and old animals. All data were received from three independent experiments.
Definition of water content of brain tissues
The water content of isolated tissue samples was determined by traditional “tissue drying” method [23]. After measuring the wet weight (w.w.) of tissue samples they were dried in oven (Factory of Medical Equipment, Odessa, Ukraine) for 24 h at 105°C for determination of dry weight (d.w.). The quantity of water in 1 g of d.w. tissue was counted by the following equation: (w.w.–d.w.)/d.w.
Counting of [3H]-ouabain receptors in the membrane
The tissue samples from in vivo experiments, which were subjected to [3H]-ouabain, were homogenized in 50 µl of 68% HNO3 solution after determination of wet and dry weights. Then 2 ml of Bray’s scintillation fluid was added and chemoluminescence of samples was quantified with 1450-MicroBeta liquid scintillation counter (Wallac, Turku, Finland). The number of [3H]-ouabain molecules’ binding with cell membranes was defined per mg of dry weight of samples. The same procedure (the definition of the number of [3H]-ouabain molecules) was performed on the tissue samples from in vitro experiments after removing them from the oven and determining wet and dry weights.
STATISTICAL ANALYSIS
Microsoft Excel and Sigma-Plot (Version 8.02A, NY, USA) were used for data analyses. The statistical significance in comparison with the sham group was calculated with Student’s t-test with the following symbols (*p<0.05; **p<0.01; ***p<0.001).
RESULTS
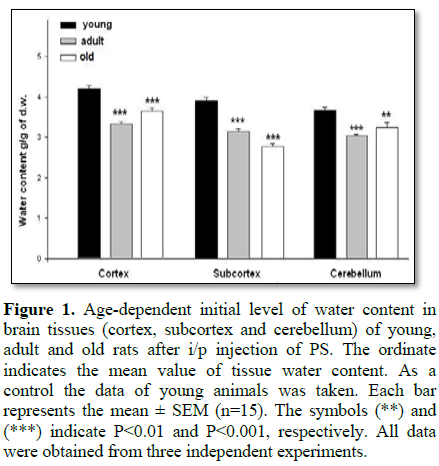
In the first series of experiments the dynamics of hydration changes in brain tissues of all animal groups was determined. On Figure 1 the variation of initial water content in tissues of three brain zones was presented in condition of active Na+/K+ pump. As can be seen, the initial water content in the tissues of young animals was higher than in adult and old ones. In addition, it is worth to note that the initial water content in cortex tissue was higher than in subcortex and cerebellum tissues in all age groups.
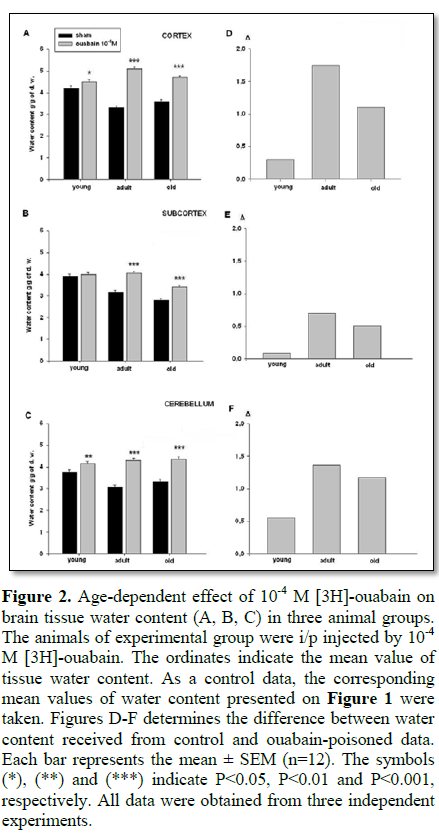
As ageing is considered as a consequence of the Na+/K+ pump dysfunction leading to decontrolling of tissue hydration, in the second series of experiments the age-dependent dynamics of Na+/K+ pump-dependent tissue hydration was studied [13,14]. The data presented on Figure 2 demonstrate that 10-4 M [3H]-ouabain (inhibitor of Na+/K+ pump) led to total hydration in all brain zones (Figures 2A-2C) as compared to control data (Figure 1). It should be noted that 10-4 M [3H]-ouabain-induced tissue hydration was less pronounced in young animals than in adult and old ones (Figures 2D-2F).
These data are in harmony with the research data [11,19,20] indicating that in early postnatal period of development, Na+/K+ pump expression is rather weak and it increases during the period of maturation. It is also worth to define that in adult animals, cortex tissue hydration is higher than in subcortex and cerebellum (Figures 2D-2F).
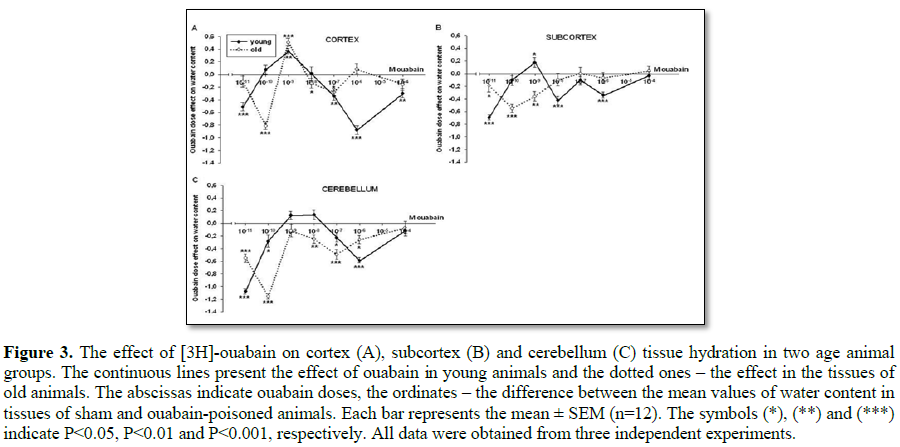
In the third series of experiments the correlation between dose-dependent [3H]-ouabain binding and brain tissue hydration was examined in all animal groups. It would let us clarify the role of Na+/K+-ATPase different isoforms (α3, α2 and α1) in age-dependent changes of tissue hydration. It should be noted that α3 isoforms are agonist to 10-11 M to 10-9 M ouabain, α2 isoforms is agonist to 10-8 M to 10-7 M ouabain and α1 isoforms are agonist to 10-6 M to 10-4 M ouabain [10].
The dose-dependent ouabain effect on tissue hydration in young and old animals is clearly seen on Figures 3A-3C, where the dependence of ouabain-induced effect is compared with the control one. On Figure 3 every point shows the difference between the mean value of water content in the tissues of control and ouabain-poisoned animals depending on the ouabain dose.
If comprehensively considering the curves of the dose-dependent ouabain effect on brain tissue hydration ranging from 10-11 M to 10-4 M, 6 different components can be identified, which are 10-11-10-10 M, 10-10 M-10-9 M, 10-9 M-10-8 M, 10-8 M-10-7 M, 10-7 M-10-6 M, 10-6 M-10-4 M. On the curves of young animals the ouabain effect in the range of ouabain at 10-11 M to 10-9 M in all investigated tissues was more sensitive and higher, especially at 10-9 M, with the expressed hydration in tissues. The similar effect (at ouabain 10-9 M) was also observed in brain cortex tissues of old animals. It must be noted that in all tissues of old animals the effect of ouabain at 10-11 M was more sensitive than at 10-11 M (the relative hydration compared to dehydration). All the other doses of ouabain in tissues of young as well as of old animals were led to dehydration effect.
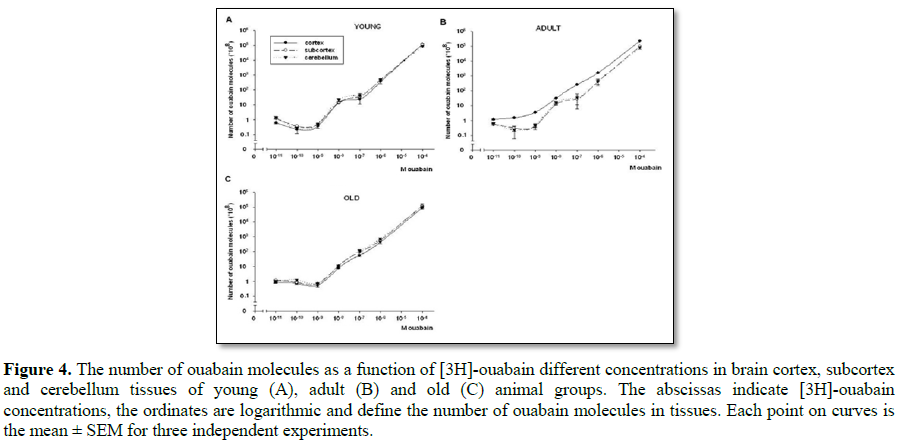
On Figures 4A-4C the data of dose-dependent [3H]-ouabain effect in brain tissues of three animal groups are shown. On the curves of ouabain binding in the tissues of young animals (Figure 4A) three parts corresponding to the next concentrations of ouabain (10-11-10-9 M, 10-9-10-7 M and 10-7-10-4 M) are obviously distinguished.
These data are confirmed by the literature data on the existence of three types of Na+/K+-ATPase α catalytic subunits (α3, α2 and α1) in excitable cell membrane [10]. As for the adult animals (Figure 4B) on their brain cortex curve of ouabain binding three part of receptors were not sharply identified, but on the curves of subcortex and cerebellum there were three different parts as in the similar curves of young animals. It must be noted that the number of ouabain molecules was much higher in brain cortex tissue than in subcortex and cerebellum ones (Figure 4B). It should be noted that in cortex tissues of adult animals the kinetics of [3H]-ouabain binding was distinctly high in the range of α3 isoforms (10-11-10-9 M). Besides, on the curve of brain cortex tissue the three parts of receptors were not distinctly expressed as in the curves of subcortex and cerebellum tissues and the α3 isoforms were more dependent on ouabain dose than α2 or α1.
On the curves of ouabain binding of old animals the dose dependency was not visible (Figure 3C), which indicates their dysfunction. As for the appearance of Na+/K+-ATPase isoforms, it must be noted that the part of α3 isoforms was highly expressed.
DISCUSSION
According to a number of studies it is a proven fact that the metabolic control of cell volume is a dynamic parameter regulating various functions of the cell [4,21-23]. It is also known that neuronal dehydration in old animals is a reliable indicator of increasing frailty, progressive deterioration in cognitive function and an overall reduction of life quality [24-27]. However, the weak knowledge on the detailed metabolic mechanism(s) controlling cell hydration in ageing is the main barrier in understanding its role in the dysfunction of cognitive processes in brain. As Na+/K+ pump dysfunction can be considered as one of the reasons for ageing, in the present study an attempt was made to find out the individual role of Na+/K+ pump different isoforms [10,11] in generation of age-dependent dehydration in brain tissues of rats.
The data obtained in the present work indicated that water content of brain cortex tissues was higher than that of subcortex and cerebellum in all age groups (Figure 1). Although the initial water content of the investigated tissues of young rats was higher than in adult ones (Figures 1A-1C) the 10-4 M ouabain-induced cell hydration (which is due to Na+/K+ pump inhibition) was significantly less than in both adult and old (i.e., mature) animals (Figure 2D-2F). It is supported by the fact that age-dependent changes of ouabain-induced brain tissue hydration had bell-shaped dynamics (young<adults>old), which is in harmony with the literature data on the reciprocal relationship between the expression of Na+/K+ pump and Na+/Ca2+ exchanger proteins in neuronal development. In prenatal and early postnatal periods the expression of Na+/Ca2+ exchanger proteins prevails, while by ageing the expression of Na+/K+ pump proteins increases, reaching to its maximum in adults and then intervenes its dysfunction in ageing [10]. From this point of view the high water content in brain tissues of young animals can be explained by higher activity of electrogenic Na+/Ca2+ exchanger [10,11], while the brain tissue dehydration in adult animals can be explained by activation of electrogenic Na+/K+ pump [5,20]. Therefore, it was predictable that 10-4 M ouabain-induced inhibition of Na+/K+ pump led to lower hydration in brain tissues of young than in mature animals (Figure 2).
The study of dose-dependent effect of ouabain on brain tissue hydration demonstrated high sensitivity to ouabain. According to these experiments it is possible to indicate that the family of Na+/K+-ATPase isoforms is not homogenic and minimum 6 components can be distinguished (Figure 3), which allows to conclude that 6 mechanisms are involved in cell volume regulation. Such heterogeneity within the family of Na+/K+-ATPase α1, α2, α3 isoforms indicates that ouabain receptors having the same affinity are functionally connected with different metabolic mechanisms involved in cell volume regulation. However, the sensitivity of α3 isoforms was pronounced in tissue hydration of both young and old animals.
In contrast to the data of ouabain dose-dependent effect on brain tissue hydration the results of dose-dependent ouabain binding with cell membrane showed only three components on curves which correspond to three Na+/K+ pump isoforms (α1, α2, α3) described in literature [9]. It is worth to note that the data on age-dependent changes of ouabain molecules’ number could not be considered as a marker for changes of expressed quantity of ouabain receptors in membrane, because of age-dependent membrane lipid composition changes, having strong modulation effect on membrane receptors affinity to agonist. However, the dose-dependent kinetics of ouabain binding with α1, α2, α3 receptors in brain tissue of three age groups of animals, could give information on age-dependent changes of their functional activity. The range of α3 receptors (agonists of 10-11 M-10-9 M ouabain) was more expressed in cortex tissues than in subcortex and cerebellum ones.
It seems extremely interesting that the process of maturation led to significant changes in ouabain binding in brain cortex, while the changes in subcortex and cerebellum of adult animals was negligible (Figures 4B and 4C), as in case of hydration (Figure 2D). It can be explained by ageing-induced increase of brain cognitive function in maturation, in which cortex has a crucial role. The disappearance of dose-dependent ouabain binding with α3 receptors (10-11-10-9 M) in the investigated tissues of aged animals could be interpreted as a marker for the dysfunction of these receptors. Such a dysfunction can be explained by the depression of their affinity in result of age-dependent increase of intracellular Ca ions [28], having inhibitory effect on Na+/K+-ATPase activity [21]. These data correspond to literature data that α3 receptors have higher affinity to intracellular Ca ions than α2 and α1 receptors [10].
CONCLUSION
The fact that the direct correlation between cell hydration and ouabain binding was absent in the above-described experiments indicates that the age-dependent depression of ouabain binding cannot only be a result of cell dehydration-induced decrease of ouabain receptors’ number in membrane, but there are other age-dependent factors leading to the decrease of ouabain receptor’s affinity to agonist. The obtained data also indicate the involvement of intracellular mechanisms in cell volume regulation with different sensitivities to ouabain.
1. Ayrapetyan SN, Arvanov VL (1979) On the mechanism of the electrogenic sodium pump dependence of membrane chemosensitivity. Comp Biochem Physiol A Physiol 64: 601-604.
2. Ayrapetyan SN, Arvanov VL, Maginyan SB, Azatyan KV (1985) Further study of the correlation between Na-pump activity and membrane chemosensitivity. Cell Mol Neurobiol 5: 231-243.
3. Ayrapetyan SN, Rychkov GY, SuleymanyanMA (1988) Effect of water flow on transmembrane ionic currents in neurons of Helix pomatia and in Squid giant axons. Comp Biochem Physiol A Physiol 89: 179-186.
4. Ayrapetyan SN (1980) On the physiological significance of pump induced cell volume changes. Adv Physiol Sci 23: 67-82.
5. Ayrapetyan SN, Suleymanyan MA, Saghyan AA, Dadalyan SS (1984) Autoregulation of the electrogenic sodium pump. Cell Mol Neurobiol 4: 367-383.
6. Kostyuk PG, Mironov SL, Doroshenko PA (1982) Energy profile of the calcium channel in the membrane of mollusc neurons. J Membrane Biol 70: 181-189.
7. Ayrapetyan S, North A (2001) Na+/K+ pump and Na+/Ca2+ exchanger as metabolic regulators and sensors for extra weak signals in neuromembrane. Mod Problems Cell Mol Biophys.
8. Sweadner KJ (1989) Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185-220.
9. Therien AG, Nestor NB, Ball WJ, Blostein R (1996) Tissue-specific versus isoform-specific differences in cation activation kinetics of the Na, K-ATPase. Am Soc Biochem Mol Biol 271: 7104-7112.
10. Juhaszova M, Blaustein MP (1997) Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci U S A 94: 1800-1805.
11. Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: Its physiological implications. Physiol Rev 79: 763-854.
12. Dipolo R, Beauge L (2006) Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol Rev 86: 155-203.
13. Fraser CL, Arieff AI (2001) Na-K-ATPase activity decreases with aging in female rat brain synaptosomes. Am J Physiol Renal Physiol 281: F674-F678.
14. Narinyan L, Ayrapetyan G, Ayrapetyan S (2012) Age-dependent magnetosensitivity of heart muscle hydration. Bioelectromagnetics 33: 452-458.
15. Krnjevic K (1974) Central actions of general anesthetics. Molecular mechanisms in general anesthesia. Churchill Livingstone, Edinburgh.
16. Haworth RA, Goknur AB, Bertroff HA (1989) Inhibition of Na-Ca exchange by general anesthetics. Circ Res 65: 1021-1028.
17. Heqimyan A, Deghoyan A, Ayrapetyan S (2011) Ketamine induced cell dehydration as a mechanism of its analgesic and anesthetic effects. J Int Dent Med Res 4: 42-49.
18. Takahashi R, Aprison M (1964) Acetylcholine content of discrete areas of the brain obtained by a near-freezing method. J Neurochem 11: 887-898.
19. Cooke KR (1978) Oubain and regulation of cellular volume in freshly prepared slices of rabbit renal cortex. J Physiol 279: 361-374.
20. Ayrapetyan SN, Suleymanyan MA (1979) On the pump-induced cell volume changes. Comp Biochem Physiol A Physiol 64: 571-575.
21. Haussinger D (1996) The role of cellular hydration in the regulation of cell function. Biochem J 313: 697-710.
22. Hoffmann EK, Lambert IH, Pedersen SF (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89: 193-277.
23. Ayrapetyan SN (2012) Cell hydration as a universal marker for detection of environmental pollution. The Environmentalist 32: 210-221.
24. Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, et al. (1994) The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell 78: 45-57.
25. Miller DH, Albert PS, Barkhof F, Francis G, Frank JA, et al. (1996) Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. US National MS Society Task Force. Ann Neurol 39: 6-16.
26. Darquie A, Poline JB, Poupon C, Saint-Jalmes H, Le Bihan D (2001) Transient decrease in water diffusion observed in human occipital cortex during visual stimulation. Natl Acad Sci U S A 98: 9391-9395.
27. Wilson MM, Morley JE (2003) Invited review: Aging and energy balance. J Appl Physiol 95: 1728-1736.
28. Khachaturyan ZS (1994) Calcium hypothesis of Alzheimer’s disease and brain aging. Ann N Y Acad Sci 747: 1-11.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Chemotherapy Research Journal (ISSN:2642-0236)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)